Watch this animation, which was designed for patients and caregivers to show how GammaTile Therapy works.
How it works
Your neurosurgeon places GammaTile(s) precisely where and when treatment will help the most—at the tumor site immediately after tumor removal.
Radiation is focused right where it is needed—where the tumor is most likely to recur.
GammaTile is designed to protect healthy tissue, minimizing radiation side effects, including hair loss.[1]
Radiation therapy occurs as you go about your daily life.

The Benefits Are Clear
Improved Local Tumor Control
For patients with recurrent meningiomas and recurrent brain metastases, the use of GammaTile Therapy demonstrated a significant delay in treatment site recurrence compared to their previous treatments.[2,3]
Improved Survival
GammaTile Therapy demonstrates a potential for improved overall survival when comparing the effectiveness of surgery plus GammaTile Therapy to other treatment modalities across different clinical studies in patients with recurrent glioblastoma (GBM).[4]
Get a head STaRT in the fight against brain tumors.
Find a GammaTile Therapy Center near you.
GammaTile Therapy: Clinical Outcomes
Improved local tumor control in patients with recurrent meningiomas and recurrent brain metastases
MENINGIOMA PATIENTS RECURRENCE FREE AT 2 YEARS[5]
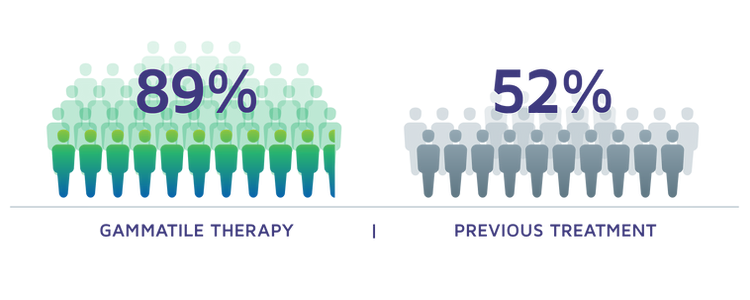
BRAIN METASTASES PATIENTS RECURRENCE FREE AT 1 YEAR[3]

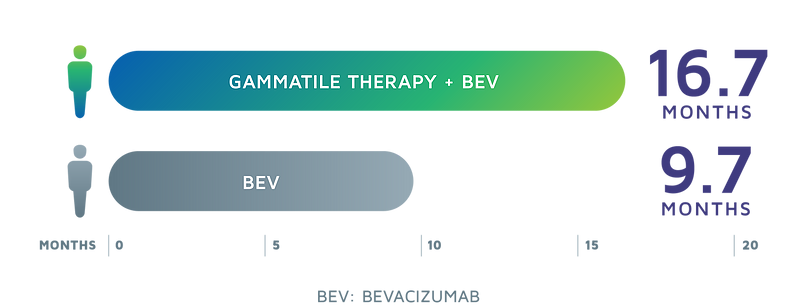
Potential to extend overall survival in patients with recurrent glioblastoma (GBM)
RECURRENT GBM PATIENTS MEDIAN OVERALL SURVIVAL[4,5]
Learn about the company that developed GammaTile Therapy.
REFERENCES:
- Brachman D, et al. Surgically Targeted Radiation Therapy: Safety Profile of Collagen Tile Brachytherapy in 79 Recurrent, Previously Irradiated Intracranial Neoplasms on a Prospective Clinical Trial. Brachytherapy 18, S35–S36 (2019).
- Brachman D, et al. Resection and permanent intracranial brachytherapy using modular, biocompatible cesium-131 implants: results in 20 recurrent, previously irradiated meningiomas. J Neurosurg 131, 1819–1828 (2019).
- Imber B et al. Salvage resec1on plus cesium-131 brachytherapy durably controls post-SRS recurrent brain metastases. J Neuro-oncol 159, 609–618 (2022).
- Gessler D, et al. GammaTile® brachytherapy in the treatment of recurrent glioblastomas. Neuro-oncology Adv 4, vdab185 (2021).
- Kutuk, T. et al. Surgically targeted radia1on therapy (STaRT) for recurrent brain metastases: Ini1al clinical experience. Brachytherapy (2023) doi:10.1016/j.brachy.2023.08.002.
